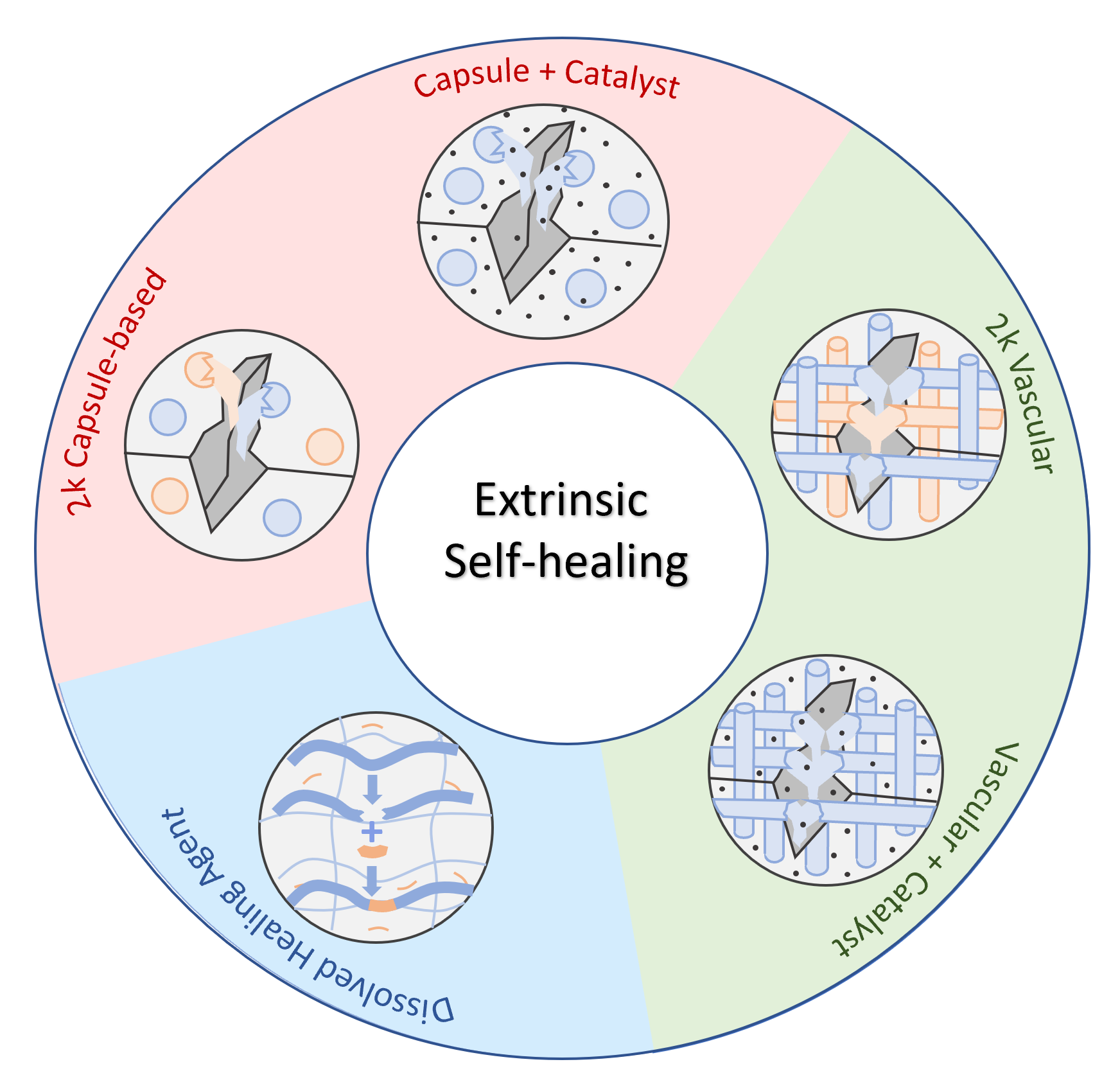
Extrinsic Self-healing
Self-healing of polymers is a relative new research topic in material and polymer science that has gained a lot attention in recent years evidenced by the steadily increasing number of publications in this field. The underlying concept and mechanisms of self-healing have been inspired from biological systems. It is an intrinsic property of all living organisms, enabling them to repair damage or injury caused by accidents, other organisms, or severe enviroments during their lifetime.
Many engineering and high-performance plastics are susceptible to mechanical damage due to their inherent brittleness. Often damage starts with microcracking induced by excessive thermal and/or mechanical stress or by cyclic loads particularly in aggressive atmospheres. These microcracks are often hidden deep in the structure and are difficult or impossible to detect, and if not repaired, will lead to lost properties or lost functionality and eventually to premature catastrophic failure.
Numerous approaches to self-healing have been proposed which can be classified into two categories:1,4 (a) intrinsic self-healing where the polymers themselves heal molecular and macroscopic damage (cracks), and (b) extrinsic self-healing in which a healing agent is pre-embedded in the polymer matrix. Self-healing materials can be further classified as autonomic, where repair is triggered by the damage itself and non-autonomic where repair is triggered by an external action such as pressure, radiation, or heat.
In extrinsic self-healing systems, the healing agent is typically sequestered from the main matrix, for example within microcapsules. When broken, these capsules release the healing agent into the damaged (cracked) zone and self-heal it by chemical reaction. This method allows for the repair of relatively large damaged areas. However, depletion of the healing agent leads to only a singular healing event.1 Another extrinsic self-healing system consists of a 3-dimensional network of capillaries or hollow fibers incorporated into a polymer matrix. Due to the interconnected microvascular structure, the healing agent in the hollow fibers can flow to the depleted areas. Thus, these composites allow for multiple healing events.
Epoxy-based systems have received the most attention in extrinsic self-healing quasi-static fracture experiments.1 A popular healing agent frequently employed is dicyclopentadiene (DCPD) which undergoes a ring-opening metathesis polymerization (ROMP) in the presence of a transition metal catalyst such as ruthenium-based Grubbs catalyst:2,3
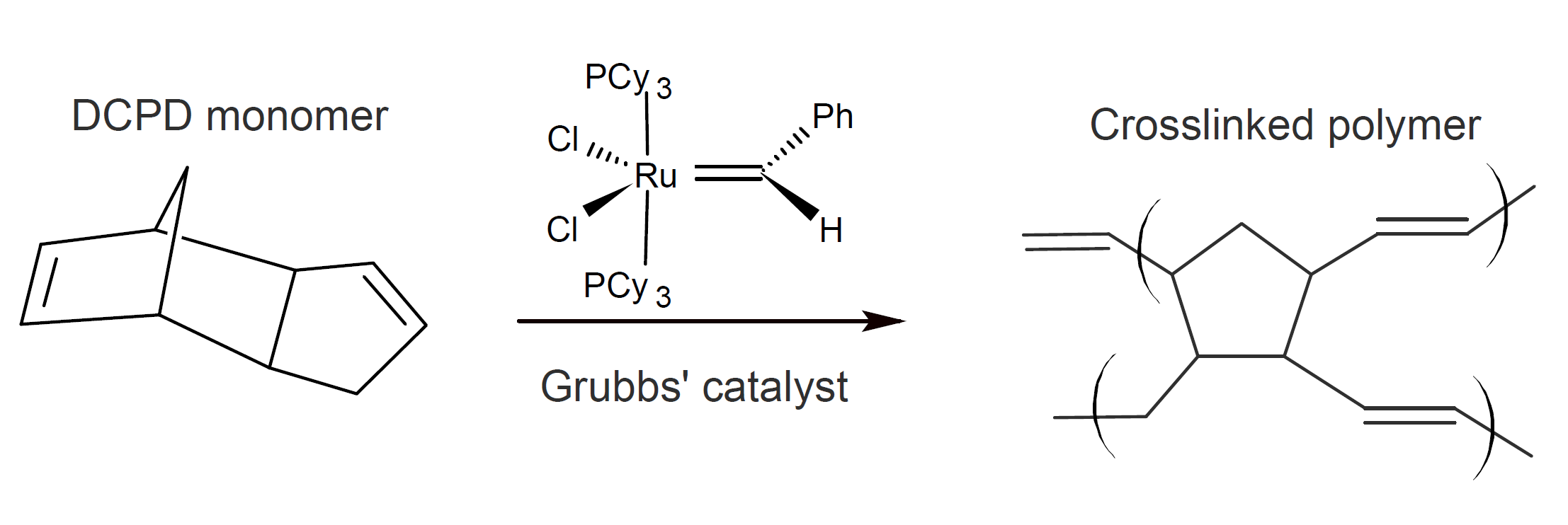
Capsules filled with DCPD are typically prepared by micro encapsulation techniques using a urea-formaldehyde shell, whereas the corresponding catalyst is dispersed in the epoxy resin matrix. A common multicapsule system consists of encapsulated epoxide and encapsulated mercaptan hardener.
Self-Healing without Encapsulation
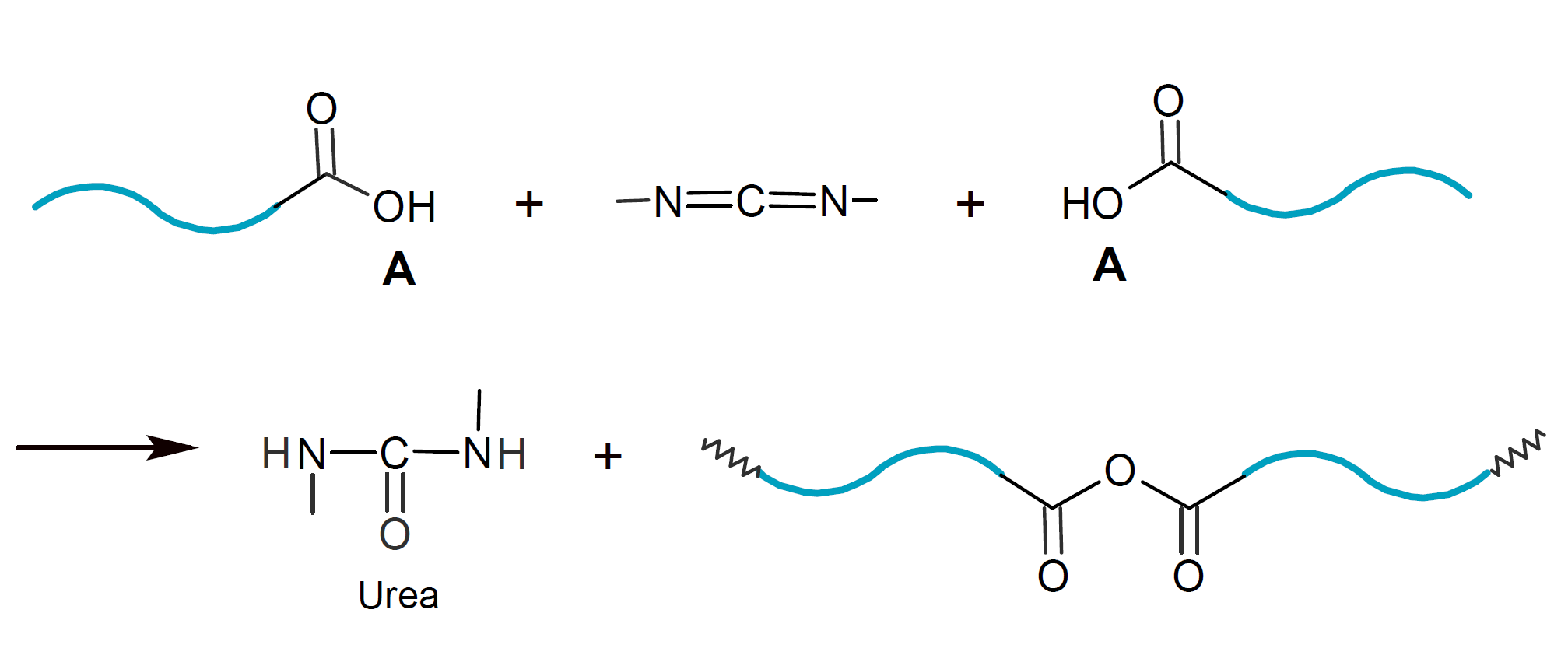
The healing methods discussed so far, require encapsulation of a healing agent. However, it is also possible to repair damage in the polymer matrix without encapsulation. This is the case, if a reactive compound and/or a catalyst is present in the polymer matrix which is able to react with the broken chain ends. This method of self-healing, for example, may be applied to polymers that are susceptible to hydrolysis such as polyesters, polyamides and polyanhydrides. Degradation occurs by diffusion of water into the amorphous regions with subsequent hydrolysis. In the case of polyanhydrides, the products of this process are oligomers with carboxylic acid chain-end functionality. These broken chains may be mended with dissolved or dispersed compounds that carry two or more functionalities that are able to react with carboxylic acid end groups. For example, the reaction with biscarbodiimides would result in the formation N-acyl urea linkages,7
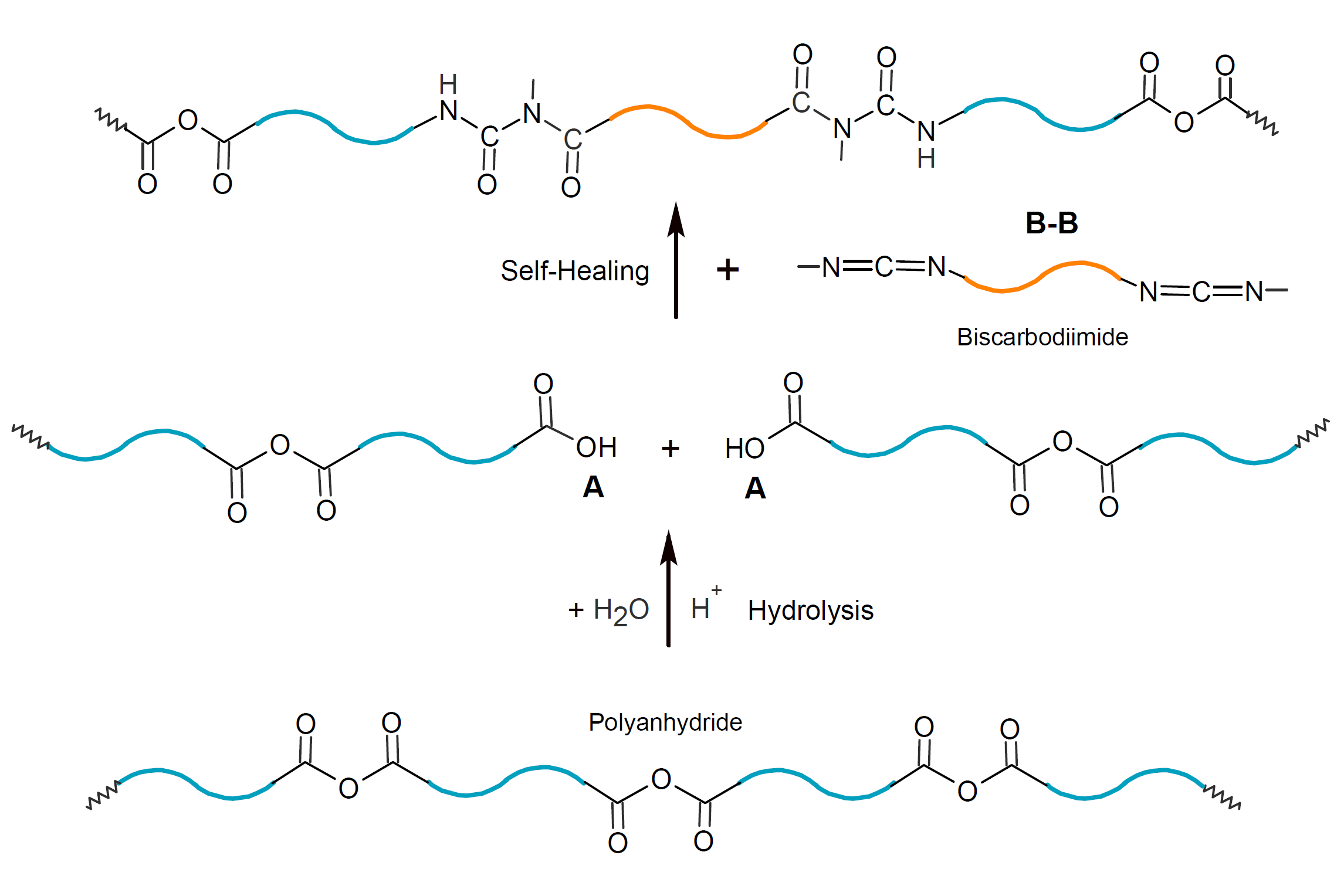
whereas the competing reaction with water produces urea. The latter reaction is very effective to bind water which greatly reduces the rate of hydrolysis.6 Thus, carbodiimides act simultaneously as stabilizers suppressing hydrolytic scission of hydrolysable polymers such as aliphatic polyesters.9
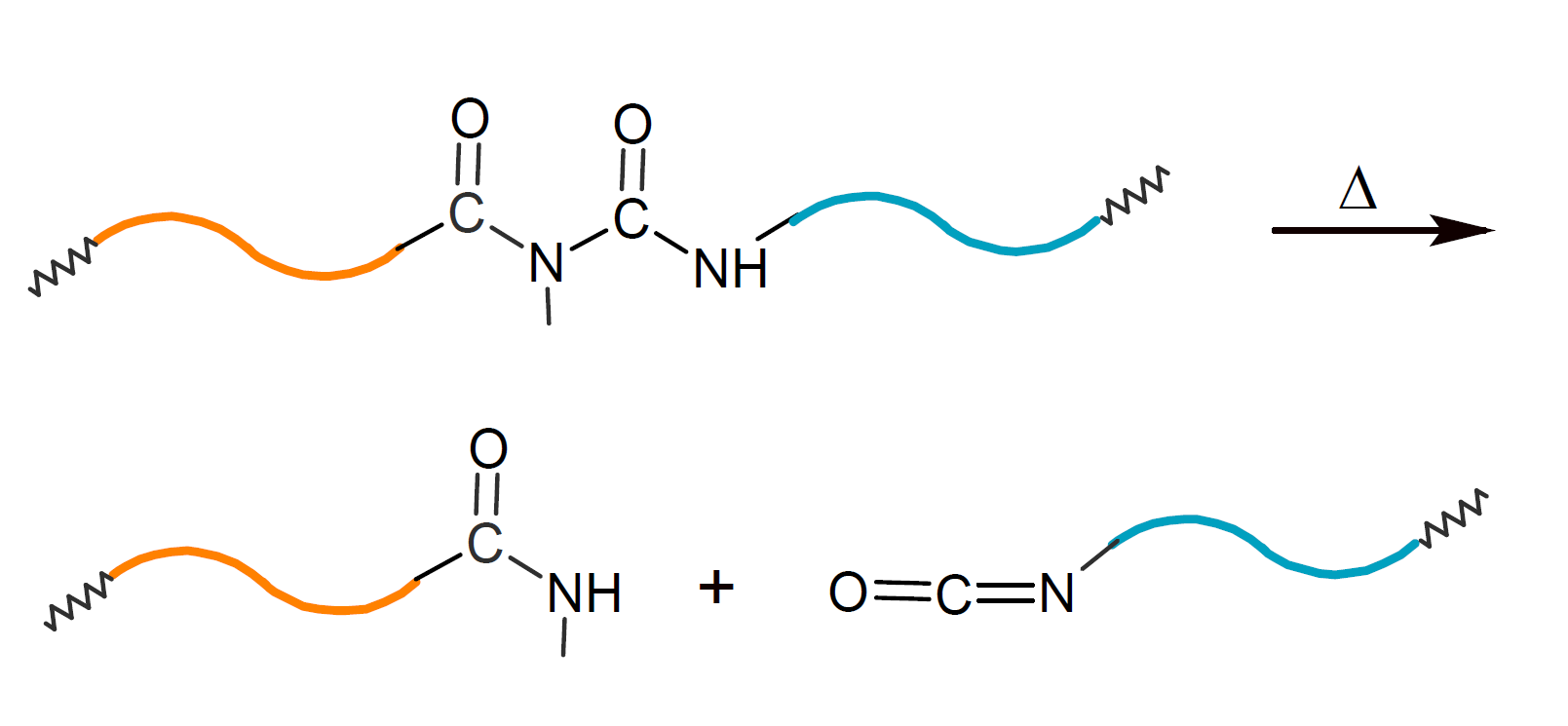
A potential drawback of the method above is the limited thermal stability of N-acyl urea linkages which decompose into amide and isocyanate groups at elevated temperatures:
Depending on the reaction conditions and concentration of carboxyl groups, carbodiimides may also form anhydride linkages, with urea as a side product:7
To avoid the formation of (volatile) reaction products and to extend the healing to other reactive groups, (multifunctional) cyclic carbodiimides may be employed.10,11 These compounds could repair damage in polyamides and polyesters caused by hydrolytic cleavage:
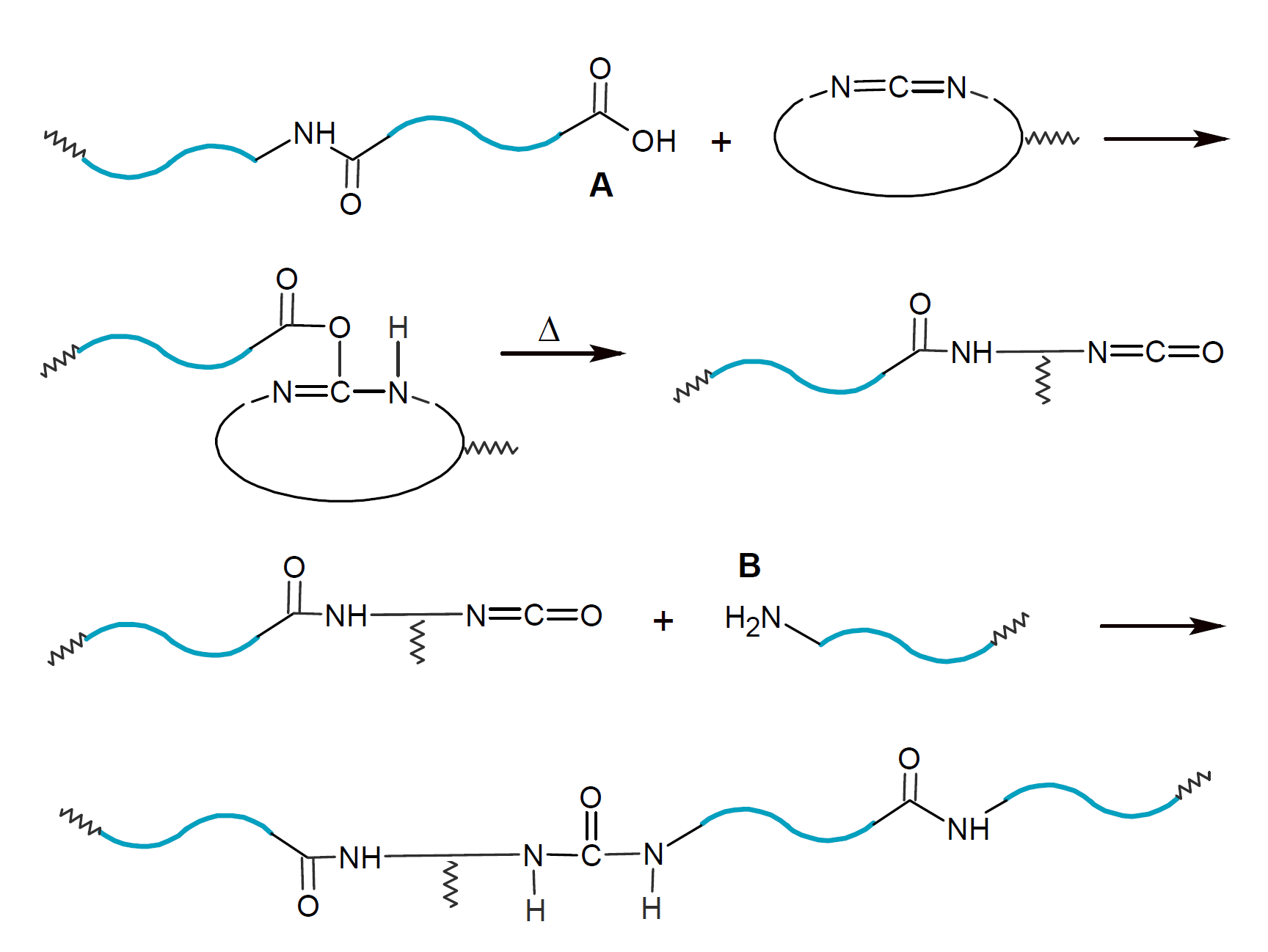
This method may be particulalry effective in preventing and healing hydrolytic cleavage of biodegradable polymers such as polylactic acid.
The healing efficiency of dispersed / dissolved (blocked) healing agents will depend on their solubility and mobility in the polymer matrix and on their reactivity, which in turn, depend on the activation and reaction temperature and type and amount of catalyst present in the polymer matrix.
Very few scientific publications on the healing efficacy of dissolved / dispersed (blocked) healing agents could be found. Two other examples are the hydrolysis and recombination of polycarbonate in the presence of sodium carbonate12 and the self-repair of poly(phenylene ether) (PPE) in the presence of a copper-complex, with the repairing agent regenerated by oxygen.13 However, in both of these examples the healing agent acts as a catalyst and not as a coreactant.
References and Notes
B.J. Blaiszik, S.L.B. Kramer, S.C. Olugebefola, J.S. Moore, N.R. Sottos, S.R. White, Annu. Rev. Mater. Res. 40, 179-211 (2010)
S.R. White, N.R. Sottos, P.H. Geubelle, J.S. Moore, M.R. Kessler, S.R. Sriram, E.N. Brown, S. Viswanathan, Nature, vol. 409, 794 - 817 (2001)
G.E. Larin, N. Bernklau, M.R. Kessler, J.C. DiCesare, Polymer Engineering and Science 46, 1804-11 (2006)
Y.C. Yuan, T. Yin, M.Z. Rong, M.Q. Zhang, eXPRESS Polym. Lett., Vol. 2, 4, 238-250 (2008)
S.K. Ghosh, Self-healing Materials: Fundamentals, Design Strategies, and Applications, Wiley-VCH, Weinheim, Germany 2009
P. Stloukal, G. Jandikova, M. Koutny, V. Sedlarik, Polymer Testing, 54, 19-28 (2016)
W. Posthumus, A. Derksen, J.A.M. van den Goorbergh, L.C.J. Hesselmans, Prog. Org. Coat. 58(2), 231-236 (2007)
J.G. Kennemur, and B.M. Novak, Polymer, 52, 1693-1710 (2011)
Carbodiimides will react with a number of other nucleophilic functionalities including alcohols, thiols, and amines which results in the formation of isourea, isothiourea, and guanidine linkages.8 These reactions are accelerated by protonation of the basic carbodiimide nitrogen making the neighboring carbon atom more susceptible to nucleophiles.
H. Ulrich, Chemistry and Technology of Carbodiimides, J. Wiley & Sons, Chichester 2007
S. Shoji and H. Suzuki, US Patent 8987440 B2, Cyclic Carbodiimide Compounds (2015)
K. Takeda, H. Unno, M. Zhang, J. Appl. Polym. Sci., 93, 920-926 (2004)
K. Takeda, M. Tanahashia, H. Unno, Sci. & Tech. of Adv. Mat. 4, 435-444 (2003)